CFDA Registration
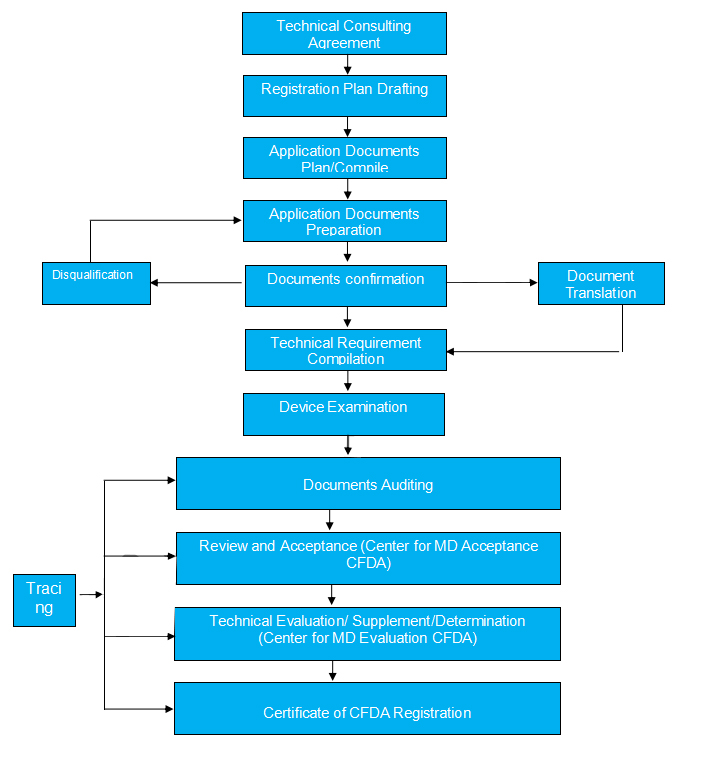
CFDA Registration Procedure (MD class III, Imported)
CFDA Registration Requirements
1.Application for MD registration
2.List of safety and validity
3.Summarize files
4.Risk Management Report
5.Research files
6.Information of the Manufacturer
7.Product Technical Request
8.Testing Report
9.Clinic evaluation
10.Users instruction/samples of least packing and label
11.Proof of Origin: Marketing approval /Authorization of Agency/ Notarization
12.Declaration of conformity
Jiushun Technical support
1. Provide guidance on registration process and technology according to CFDA regulations
2. Provide documental samples according to CFDA regulations
3. Compile Product Technical Request
4. Assist with testing
5. Provide guidance on Proof of Origin
6. Examine and verify documents and guide to adjust/submit documents to CFDA and follow up
7. Paid translation service
Why Jiushun Management?
Jiushun Management, focused on Medical Device Registration, Certification for 20 years, provided high quality service for more than 5,000 global customers.
The MD industry focus degrees, regulatory familiarity, and rich experience, determine our high efficiency and professionalism.
Our professional technical support, from the early regulations, processes guidance, documentation preparation, to testing assistance, review tracking, as well as years of MD industry resource integration, will greatly shorten the period of your product approval, and well practice our principle "Jiushun Management, create value for you. ".